Your cart is currently empty!
Tag: Effectiveness
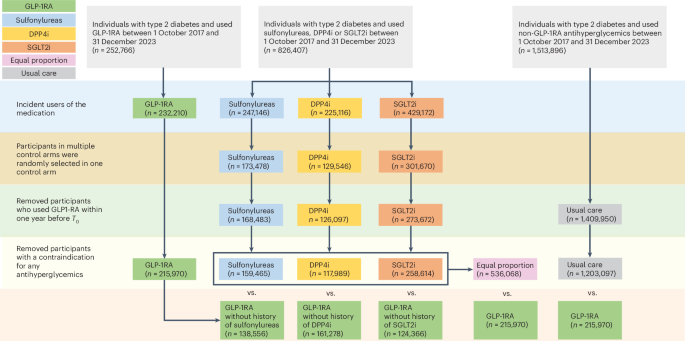
Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists

Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists
Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists

Marching for a Cause: Exploring the Effectiveness of Protest Movements
Protest movements have been a powerful tool for social change throughout history. From the civil rights movement in the 1960s to the women’s suffrage movement in the early 20th century, marching for a cause has been a way for ordinary people to make their voices heard and demand justice.In recent years, we have seen a resurgence of protest movements around the world. From the Black Lives Matter movement in the United States to the climate strikes led by young people, people are taking to the streets to demand change on a variety of issues.
But just how effective are these protest movements? Do they actually lead to meaningful change, or are they just a way for people to vent their frustrations?
There is evidence to suggest that protest movements can be effective in bringing about change. Research has shown that mass mobilization can put pressure on governments and institutions to address the concerns of protesters. For example, the civil rights movement in the United States was instrumental in ending segregation and securing voting rights for African Americans.
Protest movements can also raise awareness about important issues and mobilize public support. The women’s march in 2017, for example, drew attention to gender inequality and sparked a national conversation about women’s rights.
However, protest movements are not always successful. In some cases, governments may respond with violence or repression, leading to further unrest. Additionally, protest movements can be divisive and may not always represent the views of the entire population.
Despite these challenges, protest movements remain an important tool for social change. By raising awareness, mobilizing public support, and putting pressure on governments and institutions, protesters can make a difference and bring about meaningful change.
In conclusion, while protest movements may not always lead to immediate results, they can be an effective way for ordinary people to make their voices heard and demand justice. By marching for a cause, individuals can be part of a larger movement for social change and help create a more just and equitable society.
#Marching #Exploring #Effectiveness #Protest #Movements,how marchyorktimes
Organising Knowledge: Taxonomies, Knowledge and Organisational Effectiveness

Organising Knowledge: Taxonomies, Knowledge and Organisational Effectiveness
Price : 8.13
Ends on : N/A
View on eBay
In today’s fast-paced world, organisations are constantly inundated with vast amounts of information and data. In order to stay competitive and efficient, it is crucial for businesses to effectively organise and manage their knowledge. One key tool in achieving this is the use of taxonomies.Taxonomies are essentially classification systems that help organise information in a structured and logical manner. By categorising and grouping related concepts, taxonomies enable businesses to easily locate and retrieve the information they need. This not only saves time and effort, but also enhances decision-making and problem-solving processes.
Furthermore, taxonomies play a vital role in promoting knowledge sharing and collaboration within an organisation. By standardising terminology and creating a common framework for information, employees are better able to communicate and work together effectively. This in turn leads to increased productivity and innovation.
Ultimately, a well-designed taxonomy can greatly enhance organisational effectiveness by streamlining processes, improving information management, and fostering a culture of knowledge sharing. By investing in the development and implementation of taxonomies, businesses can gain a competitive edge in today’s knowledge-driven economy.
#Organising #Knowledge #Taxonomies #Knowledge #Organisational #Effectiveness, Data Management
AI in Classrooms Made Simple: Teacher-Friendly Guide to Integrate Artificial Intelligence to Engage students, Promote Critical Thinking, Improve Learning … , and Increase Teaching Effectiveness
Price: $4.99
(as of Dec 24,2024 14:58:44 UTC – Details)
ASIN : B0DFNRJ1KC
Publisher : Always Learning Publications (November 6, 2024)
Publication date : November 6, 2024
Language : English
File size : 1678 KB
Simultaneous device usage : Unlimited
Text-to-Speech : Enabled
Screen Reader : Supported
Enhanced typesetting : Enabled
X-Ray : Not Enabled
Word Wise : Enabled
Print length : 163 pagesCustomers say
Customers find this book provides practical classroom guides to help teachers use apps effectively. They say it’s a strong resource for educators looking to embrace AI and modernize their teaching methods. Readers describe the book as engaging and recommend it as a class book.
AI-generated from the text of customer reviews
Are you looking to incorporate artificial intelligence into your classroom but not sure where to start? Look no further! In this teacher-friendly guide, we will break down how to seamlessly integrate AI technology to engage students, promote critical thinking, improve learning outcomes, and increase teaching effectiveness.1. Engage Students: Use AI-powered tools like chatbots, virtual tutors, and personalized learning platforms to keep students actively participating in lessons. These interactive tools can provide instant feedback, quizzes, and games to make learning more engaging and fun.
2. Promote Critical Thinking: AI can help students develop critical thinking skills by analyzing data, identifying patterns, and making predictions. Encourage students to use AI tools to explore real-world problems, conduct research, and come up with creative solutions.
3. Improve Learning: AI can personalize learning experiences based on student performance, preferences, and needs. Utilize adaptive learning platforms to create individualized lesson plans, recommend resources, and track progress to ensure that every student reaches their full potential.
4. Increase Teaching Effectiveness: AI can support teachers by automating administrative tasks, grading assignments, and providing insights into student performance. By freeing up time for teachers to focus on instruction, professional development, and building relationships with students, AI can enhance teaching effectiveness and overall classroom productivity.
By following these simple steps, you can harness the power of AI to create a more engaging, interactive, and effective learning environment for your students. Embrace the future of education with artificial intelligence in the classroom!
#Classrooms #Simple #TeacherFriendly #Guide #Integrate #Artificial #Intelligence #Engage #students #Promote #Critical #Thinking #Improve #Learning #Increase #Teaching #Effectiveness
Driving Efficiency and Effectiveness: Harnessing Root Cause Analysis in Data Center Management
In today’s fast-paced and ever-evolving digital landscape, data centers play a crucial role in ensuring the smooth functioning of businesses and organizations. With the increasing amount of data being generated and processed, data center management has become a critical aspect of business operations. In order to drive efficiency and effectiveness in data center management, it is essential to harness root cause analysis.Root cause analysis is a methodical approach to identifying the underlying causes of problems and issues within a system. By digging deep into the root causes of problems, organizations can not only address immediate issues but also prevent them from recurring in the future. In the context of data center management, root cause analysis can help identify the underlying reasons for performance issues, downtime, and other operational challenges.
One of the key benefits of using root cause analysis in data center management is the ability to optimize performance and efficiency. By identifying and addressing the root causes of performance issues, organizations can improve the overall efficiency of their data center operations. This, in turn, can lead to cost savings, increased productivity, and better overall performance.
Another important aspect of using root cause analysis in data center management is the ability to enhance reliability and resilience. By identifying and addressing the root causes of downtime and other operational challenges, organizations can improve the reliability of their data center operations. This can help minimize the impact of outages and disruptions, ensuring that critical business operations continue to run smoothly.
In addition to improving efficiency and reliability, root cause analysis can also help organizations make more informed decisions about their data center infrastructure. By understanding the underlying causes of performance issues, organizations can make targeted investments in equipment, technologies, and processes that will have the greatest impact on improving overall performance.
Implementing root cause analysis in data center management requires a systematic approach. Organizations must collect and analyze data, identify patterns and trends, and collaborate across teams to identify and address root causes. By investing in the right tools and technologies, organizations can streamline the root cause analysis process and drive continuous improvement in their data center operations.
In conclusion, harnessing root cause analysis in data center management is essential for driving efficiency and effectiveness. By identifying and addressing the underlying causes of problems, organizations can optimize performance, enhance reliability, and make more informed decisions about their data center infrastructure. By investing in the right tools and technologies, organizations can ensure that their data center operations remain efficient, reliable, and resilient in the face of today’s rapidly changing digital landscape.

Optimizing Power Usage Effectiveness (PUE) Through Strategic Power Distribution in Data Centers
In today’s digital age, data centers play a critical role in storing and processing vast amounts of information. With the increasing demand for data storage and computing power, data centers are under more pressure than ever to optimize their power usage effectiveness (PUE) to reduce costs and environmental impact.One key way to improve PUE is through strategic power distribution in data centers. By carefully designing and implementing power distribution systems, data center operators can minimize energy waste and improve overall efficiency.
One important aspect of optimizing power distribution is ensuring that power is delivered efficiently to the servers and other equipment in the data center. This can be achieved by using high-efficiency power distribution units (PDUs) and transformers, as well as implementing hot aisle/cold aisle containment systems to minimize air mixing and reduce cooling requirements.
Another key consideration in optimizing power distribution is balancing the load across the different phases of the electrical system. By evenly distributing the load, data center operators can prevent overloading and maximize the capacity of the electrical system. This can be achieved through careful planning and monitoring of power usage, as well as using power management tools to optimize load balancing.
In addition to optimizing power distribution within the data center, it is also important to consider the source of the power itself. Using renewable energy sources, such as solar or wind power, can help reduce the carbon footprint of the data center and improve overall sustainability.
Overall, optimizing power usage effectiveness through strategic power distribution is crucial for data centers looking to reduce costs, improve efficiency, and minimize environmental impact. By carefully designing and implementing power distribution systems, data center operators can achieve significant energy savings and create a more sustainable operation.
Understanding Power Usage Effectiveness (PUE) and Its Impact on Data Center Energy Efficiency
Understanding Power Usage Effectiveness (PUE) and Its Impact on Data Center Energy EfficiencyIn today’s digital age, data centers play a crucial role in storing, processing, and managing vast amounts of data. With the increasing demand for data storage and processing capabilities, data centers have become an essential part of the modern infrastructure. However, the energy consumption of data centers has also been on the rise, leading to concerns about their environmental impact and energy efficiency.
One of the key metrics used to measure the energy efficiency of data centers is Power Usage Effectiveness (PUE). PUE is a ratio that indicates how much of the total energy consumed by a data center is used for IT equipment, as opposed to supporting infrastructure such as cooling systems and lighting. The lower the PUE value, the more efficient the data center is in terms of energy usage.
PUE is calculated by dividing the total energy consumption of the data center by the energy consumption of the IT equipment alone. A perfect PUE value would be 1.0, indicating that all the energy consumed is used by the IT equipment. However, in reality, most data centers have PUE values higher than 1.0, as a portion of the energy is used for cooling, lighting, and other non-IT equipment.
The impact of PUE on data center energy efficiency is significant. A lower PUE value means that a higher percentage of the energy consumed is used for actual computing tasks, resulting in reduced energy costs and a smaller environmental footprint. By improving energy efficiency and lowering PUE values, data center operators can reduce their carbon footprint and operating costs.
There are several strategies that data center operators can implement to improve energy efficiency and reduce PUE values. These include using energy-efficient IT equipment, optimizing cooling systems, implementing virtualization and consolidation techniques, and utilizing renewable energy sources. By adopting these strategies, data center operators can not only improve their energy efficiency but also contribute to a more sustainable and environmentally friendly operation.
In conclusion, understanding Power Usage Effectiveness (PUE) and its impact on data center energy efficiency is crucial for data center operators looking to reduce their energy consumption and environmental impact. By monitoring and optimizing PUE values, data center operators can improve their energy efficiency, lower operating costs, and contribute to a more sustainable future.
Maximizing Efficiency and Effectiveness in Data Center Backup and Recovery Operations
In today’s fast-paced digital world, data center backup and recovery operations are more critical than ever. With the increasing amount of data being generated and stored by organizations, ensuring that this data is backed up and recoverable in case of a disaster is essential for business continuity.Maximizing efficiency and effectiveness in data center backup and recovery operations is key to ensuring that data is protected and accessible when needed. There are several strategies that organizations can implement to improve their backup and recovery processes.
One important step in maximizing efficiency is implementing a comprehensive backup strategy that includes regular backups of all critical data. This includes not only data stored on servers, but also data stored on employee devices and in cloud applications. By ensuring that all data is backed up regularly, organizations can minimize the risk of data loss in the event of a disaster.
Another key strategy for maximizing efficiency is leveraging automation tools to streamline backup and recovery operations. Automation can help to reduce human error and ensure that backups are performed consistently and on schedule. By implementing automation tools, organizations can free up valuable IT resources to focus on other tasks, while also improving the reliability and speed of backup and recovery operations.
In addition to automation, organizations should also regularly test their backup and recovery processes to ensure that they are working as intended. Testing can help to identify any potential issues or weaknesses in the backup strategy, allowing organizations to make necessary adjustments before a disaster occurs. By regularly testing backup and recovery processes, organizations can ensure that they are prepared to quickly recover data in the event of a disaster.
Finally, organizations should consider implementing a multi-tiered backup strategy that includes both on-premises and cloud-based backup solutions. By utilizing both on-premises and cloud-based backups, organizations can ensure that their data is protected in multiple locations and is accessible even in the event of a physical disaster at the data center. This multi-tiered approach can help to maximize the effectiveness of backup and recovery operations and ensure that data is always available when needed.
In conclusion, maximizing efficiency and effectiveness in data center backup and recovery operations is essential for ensuring business continuity and protecting critical data. By implementing a comprehensive backup strategy, leveraging automation tools, regularly testing backup processes, and utilizing a multi-tiered backup approach, organizations can improve the reliability and speed of their backup and recovery operations. By prioritizing data protection and implementing best practices in backup and recovery, organizations can minimize the risk of data loss and ensure that their data is always accessible when needed.
