Your cart is currently empty!
Tag: GLP1
Josh Gad Reveals He’s Lost 40 Lbs on GLP-1 Medication

Josh Gad Theo Wargo/Getty ImagesJosh Gad is opening up about the weight loss success he’s had with GLP-1 medication — and sharing some of the conflicted feelings he’s had along the way.
“I’m on a GLP-1 … this is the first time I’ve opened up about this,” the actor, 43, revealed during the Monday, January 27, episode of Dax Shepard’s “Armchair Expert” podcast. “It has suppressed, in a great way, that noise. … When I wake up, I feel hunger pains — and so much of that is psychological, right? And what this does is it takes away that signal.”
GLP-1 medications, which include Ozempic and Mounjaro, are a class of drugs originally developed to treat diabetes. They can also be prescribed for weight loss in conjunction with diet and exercise.
Gad revealed on Monday that he lost 40 pounds on the first GLP-1 he tried but had to change medications because he developed diverticulitis, an inflammation of small pouches that build in the colon.
“It is a miracle drug,” he said. “I was really bummed out because it was working incredibly for me and I had to switch.”
The Frozen star noted that his medication doesn’t mean he can eat whatever he wants or skip the gym.

Josh Gad in 2018 and 2025. WireImage; Getty Images“I’m figuring out this new one, and it is life-changing, but it also doesn’t negate the fact that it can’t be in the place of having a healthy relationship with food,” he explained. “It can’t be in the place of having a healthy relationship with exercise. … I’m having my own journey with it. Sometimes I feel like I’m cheating myself by doing this.”
Gad went on to admit that he has worried about what his weight loss will mean for his career.
“I’ve always been the funny fat guy. Can I be the funny skinny guy? Can I be the hot leading man?” he wondered. “I don’t know that people would accept me as those things.”
His kids, however, are the main thing driving his journey. The comedian shares daughters Ava, 14, and Isabella, 10, with wife Ida Darvish.
“I’m not as worried about [my career] because my primary goal is, I want to be there for my kids,” Gad said. “Everything else is bulls—.”
Gad also said that Darvish, 49, is concerned he won’t address the underlying issues that have caused past weight gain, noting that she’s “not thrilled” he’s taking the medication.
Josh Gad, known for his roles in movies like “Frozen” and “Beauty and the Beast,” has recently revealed that he has lost 40 lbs thanks to GLP-1 medication. The actor took to social media to share his weight loss journey, expressing his excitement and gratitude for the progress he has made.GLP-1 medication, also known as glucagon-like peptide-1 receptor agonists, is commonly used to treat type 2 diabetes but has also been shown to aid in weight loss. Josh Gad’s success with this medication serves as an inspiration to others who may be struggling with their own weight loss goals.
Fans of the actor have been quick to congratulate him on his achievement, praising his dedication and determination. Josh Gad’s openness about his weight loss journey is not only commendable but also serves as a reminder that with the right support and resources, anyone can achieve their health and wellness goals.
Congratulations to Josh Gad on his impressive weight loss journey – we can’t wait to see where his newfound health and vitality take him next!
Tags:
- Josh Gad weight loss journey
- GLP-1 medication success story
- Celebrity weight loss transformation
- How Josh Gad lost 40 lbs
- GLP-1 treatment for weight loss
- Josh Gad health update
- Weight loss progress with GLP-1 medication
- Celebrity health news
- Josh Gad’s weight loss secret
- GLP-1 medication and weight loss benefits
#Josh #Gad #Reveals #Hes #Lost #Lbs #GLP1 #Medication
Allurion Launches Breakthrough Study Combining Gastric Balloon with GLP-1 to Preserve Muscle Mass
Allurion Technologies (NYSE: ALUR) has announced plans to start a clinical study combining its Allurion Program with GLP-1 agonists to optimize muscle mass during weight loss treatment. The initiative addresses a significant concern with GLP-1 therapy, which has shown approximately 40% reduction in lean mass as a proportion of total weight lost.
Previous studies of the Allurion Balloon combined with their Virtual Care Suite have demonstrated promising results: In a study of 571 patients, participants gained 5.6% in lean body mass while losing 14% of total body weight over four months. Another study with 167 patients showed a 15.7% weight reduction with no muscle mass loss.
The company aims to prove that combining their balloon technology with GLP-1 therapy could become the gold standard for obesity care by enabling significant weight loss while improving muscle mass and body composition.
Allurion Technologies (NYSE: ALUR) ha annunciato piani per avviare uno studio clinico che combina il suo Programma Allurion con agonisti del GLP-1 per ottimizzare la massa muscolare durante il trattamento per la perdita di peso. Questa iniziativa affronta una preoccupazione significativa con la terapia GLP-1, che ha mostrato una riduzione di circa il 40% della massa magra come proporzione del peso totale perso.
Studi precedenti sul pallone Allurion combinato con la loro Virtual Care Suite hanno dimostrato risultati promettenti: in uno studio di 571 pazienti, i partecipanti hanno guadagnato il 5,6% di massa corporea magra mentre perdevano il 14% del peso corporeo totale in quattro mesi. Un altro studio con 167 pazienti ha mostrato una riduzione del peso del 15,7% senza perdita di massa muscolare.
L’azienda mira a dimostrare che la combinazione della loro tecnologia del pallone con la terapia GLP-1 potrebbe diventare lo standard d’oro per la cura dell’obesità, consentendo una significativa perdita di peso mentre si migliora la massa muscolare e la composizione corporea.
Allurion Technologies (NYSE: ALUR) ha anunciado planes para iniciar un estudio clínico que combina su Programa Allurion con agonistas de GLP-1 para optimizar la masa muscular durante el tratamiento para la pérdida de peso. Esta iniciativa aborda una preocupación significativa con la terapia GLP-1, que ha mostrado una reducción de aproximadamente el 40% en la masa magra como proporción del peso total perdido.
Estudios previos sobre el Globo Allurion combinado con su Virtual Care Suite han demostrado resultados prometedores: en un estudio de 571 pacientes, los participantes ganaron un 5,6% en masa corporal magra mientras perdían un 14% del peso corporal total en cuatro meses. Otro estudio con 167 pacientes mostró una reducción del peso del 15,7% sin pérdida de masa muscular.
La empresa tiene como objetivo demostrar que la combinación de su tecnología de globo con la terapia de GLP-1 podría convertirse en el estándar de oro para el tratamiento de la obesidad al permitir una pérdida de peso significativa mientras se mejora la masa muscular y la composición corporal.
올루리온 테크놀로지스 (NYSE: ALUR)는 체중 감소 치료 중 근육량을 최적화하기 위해 Allurion 프로그램과 GLP-1 작용제를 결합한 임상 연구를 시작할 계획을 발표했습니다. 이 이니셔티브는 GLP-1 요법과 관련된 심각한 우려를 다루고 있으며, 이는 총 체중 감소의 약 40%가 제지방으로 감소하는 것으로 나타났습니다.
올루리온 풍선과 그들의 가상 케어 스위트를 결합한 이전 연구들은 유망한 결과를 보여주었습니다: 571명의 환자를 대상으로 한 연구에서 참가자들은 4개월 동안 총 체중의 14%를 잃는 동안 5.6%의 제지방량을 증가시켰습니다. 167명의 환자를 대상으로 한 또 다른 연구에서는 근육량 손실 없이 15.7%의 체중 감소가 나타났습니다.
회사는 풍선 기술과 GLP-1 요법의 조합이 체중 감소를 유도하면서 근육량과 신체 구성 개선을 가능하게 하여 비만 치료의 금본위제가 될 수 있음을 입증하는 것을 목표로 하고 있습니다.
Allurion Technologies (NYSE: ALUR) a annoncé des plans pour commencer une étude clinique combinant son Programme Allurion avec des agonistes du GLP-1 afin d’optimiser la masse musculaire durant le traitement de la perte de poids. Cette initiative répond à une préoccupation significative liée à la thérapie GLP-1, qui a montré une réduction d’environ 40% de la masse maigre par rapport au poids total perdu.
Des études antérieures sur le ballon Allurion combiné avec leur Virtual Care Suite ont démontré des résultats prometteurs : dans une étude de 571 patients, les participants ont gagné 5,6% en masse corporelle maigre tout en perdant 14% de leur poids corporel total en quatre mois. Une autre étude avec 167 patients a montré une réduction de poids de 15,7% sans perte de masse musculaire.
L’entreprise vise à prouver que la combinaison de sa technologie de ballon avec la thérapie GLP-1 pourrait devenir la norme en matière de soins contre l’obésité, en permettant une perte de poids significative tout en améliorant la masse musculaire et la composition corporelle.
Allurion Technologies (NYSE: ALUR) hat Pläne angekündigt, eine klinische Studie zu starten, die sein Allurion-Programm mit GLP-1-Agonisten kombiniert, um die Muskelmasse während der Gewichtsabnahme zu optimieren. Diese Initiative spricht ein erhebliches Anliegen in Bezug auf die GLP-1-Therapie an, die eine Reduzierung von etwa 40% der fettfreien Masse als Anteil des insgesamt verlorenen Gewichts gezeigt hat.
Frühere Studien zum Allurion-Ballon in Kombination mit ihrem Virtual Care Suite haben vielversprechende Ergebnisse gezeigt: In einer Studie mit 571 Patienten gewannen die Teilnehmer 5,6% an fettfreier Körpermasse, während sie in vier Monaten 14% ihres Gesamtkörpergewichts verloren. Eine weitere Studie mit 167 Patienten zeigte eine Gewichtsreduktion von 15,7%, ohne dass es zu einem Verlust der Muskelmasse kam.
Das Unternehmen zielt darauf ab, nachzuweisen, dass die Kombination seiner Ballontechnologie mit der GLP-1-Therapie zum Goldstandard in der Adipositasbehandlung werden kann, indem sie eine signifikante Gewichtsabnahme bei gleichzeitiger Verbesserung der Muskelmasse und der Körperzusammensetzung ermöglicht.
Positive
- Previous studies showed 14% total weight loss with 5.6% lean mass gain over 4 months
- Another study demonstrated 15.7% weight reduction without muscle mass loss
- Potential to address significant unmet need in GLP-1 therapy market
Insights
This strategic initiative by Allurion represents a potentially game-changing development in the rapidly expanding obesity treatment market. The company is targeting a critical weakness in GLP-1 treatments – the significant loss of lean muscle mass, which affects approximately 40% of total weight lost during therapy.
The preliminary data is particularly compelling: Allurion’s existing studies show patients achieving 14-15.7% total weight loss while either maintaining or increasing muscle mass – a stark contrast to the muscle wasting observed with GLP-1 treatments alone. In one significant study of 571 patients, subjects achieved a 5.6% increase in lean body mass while losing weight, suggesting a superior metabolic outcome.
This positions Allurion to potentially capture a significant share of the complementary treatment market for GLP-1 users, estimated to reach $100 billion by 2030. The market opportunity is substantial, considering that:
- Muscle preservation during weight loss is important for long-term metabolic health and weight maintenance
- The combination therapy could become a preferred treatment protocol for healthcare providers concerned about muscle wasting in GLP-1 patients
- This approach could differentiate Allurion from other weight loss device manufacturers and position them as a leader in comprehensive obesity care
The success of this clinical study could significantly enhance Allurion’s market position and drive adoption of their program among the growing population of GLP-1 users. However, investors should note that this announcement represents an intention to initiate studies and actual clinical validation will require time and substantial investment before potential commercialization.
NATICK, Mass. –(BUSINESS WIRE)–
Allurion Technologies, Inc. (“Allurion” or the “Company”) (NYSE: ALUR), a company dedicated to ending obesity, today announced its intention to initiate a clinical study on the combination of the Allurion Program with GLP-1 agonists to improve muscle mass and overall body composition.Previous studies in patients undergoing GLP-1 therapy have demonstrated reductions in lean mass of approximately
40% as a proportion of total weight lost.1 In contrast, in previous studies, patients treated with the Allurion Balloon in combination with the Allurion Virtual Care Suite have demonstrated strong patient outcomes in which patients lose weight while maintaining, and in some cases, increasing, muscle mass. In one study of 571 patients, patients treated with the Allurion Balloon gained5.6% in lean body mass while losing14% of their total body weight over four months.2 In another study of 167 patients, patients treated with the Allurion Balloon experienced a weight reduction of15.7% with no change in muscle mass.“Reductions in lean mass and muscle wasting are significant unmet needs in the GLP-1 space, and our early data suggests that we may have a powerful tool in achieving more metabolically healthy weight loss,” said Dr. Shantanu Gaur, Founder and CEO of Allurion. “The goal of our study would be to prove that, by combining the Allurion Balloon and Allurion Virtual Care Suite with GLP-1 therapy, patients can lose significant weight while increasing muscle mass and improving overall body composition. We are optimistic that this would be a significant addition to the possibilities of GLP-1 therapies and, if proven, could become the gold standard for obesity care.”
About Allurion
Allurion is dedicated to ending obesity. The Allurion Program is a weight-loss platform that combines the Allurion Gastric Balloon, the world’s first and only swallowable, procedure-lessTM gastric balloon for weight loss, the Allurion Virtual Care Suite, including the Allurion Mobile App for consumers and Allurion Insights for healthcare providers featuring the Iris AI Platform, and the Allurion Connected Scale. The Allurion Virtual Care Suite is also available to providers separately from the Allurion Program to help customize, monitor, and manage weight-loss therapy for patients regardless of their treatment plan. The Allurion Gastric Balloon is an investigational device in
the United States .For more information about Allurion and the Allurion Virtual Care Suite, please visit www.allurion.com.
Forward-Looking Statements
This press release contains certain forward-looking statements within the meaning of the
U.S. federal and state securities laws. These forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “consider,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “target,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” “if,” and similar expressions and include statements regarding Allurion’s plan to initiate a clinical study to focus on the combination of the Allurion Program and GLP-1s designed to improve retention of muscle mass and overall body composition, the Allurion Program being a powerful tool in achieving more metabolically healthy weight loss, the ability of the study to demonstrate that the combination of the Allurion Program and GLP-1s will allow patients to lose significant weight while increasing muscle mass and improving overall body composition, the combination being a powerful addition to the standard of care if such study is initiated and completed, and other statements about future events that reflect the current beliefs and assumptions of Allurion’s management based on information currently available to them and, as a result, are subject to risks and uncertainties. Forward-looking statements are predictions, projections and other statements about future events that reflect the current beliefs and assumptions of Allurion’s management based on information currently available to them and, as a result, are subject to risks and uncertainties. Many factors could cause actual future results or developments to differ materially from the forward-looking statements in this press release, including but not limited to (i) the ability of Allurion to obtain regulatory approval for and successfully commercialize the Allurion Program, (ii) the timing of and results from its clinical studies and trials, (iii) the evolution of the markets in which Allurion competes and the rise of GLP-1 drugs, (iv) the ability of Allurion to defend its intellectual property and satisfy regulatory requirements, (v) the impact of the COVID-19 pandemic,Russia –Ukraine war and Israel-Hamas war on Allurion’s business, (vi) Allurion’s expectations regarding its market opportunities, (vii) the outcome of any legal proceedings against Allurion, (viii) the risk of economic downturns and a changing regulatory landscape in the highly competitive industry in which Allurion operates, and (ix) the ability of Allurion to obtain sufficient funding to initiate and/or complete any clinical studies that demonstrate positive results. The foregoing list of factors is not exhaustive. You should carefully consider the foregoing factors and the other risks and uncertainties described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K filed on March 26, 2026 and Amendment No. 1 thereto filed on April 29, 2024, the Company’s Quarterly Report on Form 10-Q filed on November 13, 2024 and other documents filed by Allurion from time to time with theU.S. Securities and Exchange Commission. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and Allurion assumes no obligation and does not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. Allurion does not give any assurance that it will achieve its expectations._____________________________
1 Wilding et al. NEJM. 2021, 384, 989-1002; 10.1056/NEJMoa2032183
2 Dejeu et al. Clin. Pract. 2024, 14(3), 765-778; https://doi.org/10.3390/clinpract14030061View source version on businesswire.com: https://www.businesswire.com/news/home/20250123667059/en/
Global Media
Hannah Lindberg
hlindberg@allurion.comInvestor Contact
Mike Cavanaugh, Investor Relations
ICR Westwicke
(617) 877-9641
mike.cavanaugh@westwicke.comSource: Allurion Technologies, Inc.
FAQ
What are the results of Allurion’s (ALUR) previous weight loss studies?
Previous studies showed patients lost 14% of total body weight while gaining 5.6% lean body mass over four months. A separate study demonstrated 15.7% weight reduction with no muscle mass loss.
How does Allurion’s (ALUR) approach differ from standard GLP-1 therapy?
While GLP-1 therapy typically results in 40% lean mass reduction as a proportion of total weight lost, Allurion’s program has demonstrated weight loss while maintaining or increasing muscle mass.
What is the goal of Allurion’s (ALUR) new clinical study?
The study aims to prove that combining the Allurion Balloon and Virtual Care Suite with GLP-1 therapy can achieve significant weight loss while increasing muscle mass and improving body composition.
How much lean mass is typically lost during GLP-1 therapy compared to Allurion’s (ALUR) program?
GLP-1 therapy typically results in about 40% lean mass reduction, while Allurion’s program has shown the ability to maintain or increase muscle mass during weight loss.
Allurion Launches Breakthrough Study Combining Gastric Balloon with GLP-1 to Preserve Muscle MassAllurion, a leader in non-surgical weight loss solutions, has announced the launch of a groundbreaking study that combines their Elipse gastric balloon with GLP-1 receptor agonists to help preserve muscle mass during weight loss. This innovative approach aims to address one of the biggest concerns with traditional weight loss methods – the loss of muscle mass, which can have negative effects on metabolism and overall health.
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes and have been shown to have positive effects on weight loss and metabolic health. By combining these medications with the Elipse gastric balloon, Allurion hopes to provide a comprehensive solution that not only helps patients lose weight, but also helps them maintain lean muscle mass and metabolic health.
The study will involve a group of participants who will undergo treatment with the Elipse gastric balloon and GLP-1 receptor agonists for a period of six months. Researchers will measure changes in body composition, muscle mass, metabolic markers, and overall health outcomes to evaluate the effectiveness of this novel approach.
Allurion is excited to be at the forefront of this research and hopes that the results of this study will provide valuable insights into the potential benefits of combining gastric balloons with GLP-1 receptor agonists for weight loss and muscle preservation. Stay tuned for updates on this exciting study and the potential impact it could have on the field of weight loss and metabolic health.
Tags:
Allurion, gastric balloon, GLP-1, muscle mass preservation, weight loss study, obesity treatment, innovative research, gastric balloon technology, muscle mass maintenance, metabolic health, Allurion study findings
#Allurion #Launches #Breakthrough #Study #Combining #Gastric #Balloon #GLP1 #Preserve #Muscle #Mass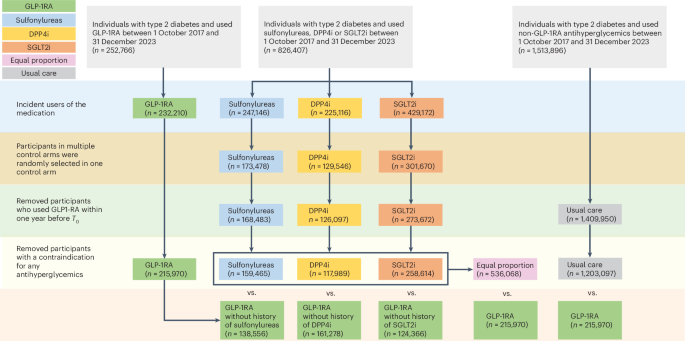
Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists

Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists

Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists
Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists
Built by Nature Akkermansia Muciniphila Probiotic Supplement – 1 Billion AFU – Supports GLP-1, Immune & Digestive Gut Health – 30 Delayed Release Blister Packed Capsules
Price:$29.99– $14.72
(as of Dec 14,2024 01:14:50 UTC – Details)
Discover the greatest innovation in probiotics with Built By Nature’s Akkermansia Probiotic Supplement. This rare and revolutionary probiotic supports the production of GLP-1, supporting digestion unlike any other probiotic before. Each capsule is packed with 1 Billion AFU Akkermansia muciniphila Akk11, known for its unique ability to nourish the gut lining and support a healthy mucosal barrier. This contributes to better digestion and immune health. Our advanced delayed release capsules are engineered to bypass the harsh environment of stomach acid, ensuring the probiotics arrive intact in the intestines where they can thrive and deliver maximum benefits. We have meticulously sealed each capsule in aluminum blister packaging to better protect the Akkermansia probiotics to ensure effectiveness. For men and women take one (1) capsule daily with water and a meal.
Package Dimensions : 6.3 x 4.37 x 1.46 inches; 1.76 ounces
Date First Available : April 29, 2024
Manufacturer : Built By Nature
ASIN : B0D2YDZNHQ
Country of Origin : China1 Billion AFU: Each capsule contains 1 Billion AFU of live strain Akkermansia muciniphila Akk11, an innovative next generation probiotic strain known for its unique ability to nourish the gut lining.
GLP-1 Support: Built By Nature’s Akkermansia Probiotic is designed to naturally support the production of GLP-1.
Immune and Digestive Support: Akkermansia is known to help repair the gut lining, boosting immune health and digestion.
Delayed Release Capsules: Utilizing stomach acid resistant capsules and blister packaging helps ensure the probiotics stay protected, allowing the Akkermansia to reach your intestinal tract intact for maximum effectiveness. For men and women take one (1) capsule daily with water and a meal.
Quality and Purity Assured: Rigorously tested and certified for quality, we are committed to delivering the purest, safest, and most effective probiotics without compromise.
Introducing our latest innovation in gut health: Built by Nature Akkermansia Muciniphila Probiotic Supplement. This powerful supplement contains 1 Billion AFU of the beneficial bacteria Akkermansia Muciniphila, known for its ability to support GLP-1, immune function, and digestive health.Our formula is specially designed with delayed-release capsules to ensure the probiotics reach your gut intact, where they can work their magic. Each blister pack contains 30 capsules, providing a month’s supply of gut-boosting goodness.
Say goodbye to digestive issues and hello to a healthier gut with Built by Nature Akkermansia Muciniphila Probiotic Supplement. Try it today and experience the benefits for yourself!
#Built #Nature #Akkermansia #Muciniphila #Probiotic #Supplement #Billion #AFU #Supports #GLP1 #Immune #Digestive #Gut #Health #Delayed #Release #Blister #Packed #Capsules
Akkermansia Probiotic Supplement with Prebiotic Inulin Fiber GLP-1 Production

Akkermansia Probiotic Supplement with Prebiotic Inulin Fiber GLP-1 Production
Price : 23.62
Ends on : N/A
View on eBay
Are you looking to improve your gut health and support weight management? Look no further than Akkermansia Probiotic Supplement with Prebiotic Inulin Fiber!Akkermansia is a beneficial bacteria that has been shown to support a healthy gut microbiome and improve metabolic health. By taking a daily probiotic supplement containing Akkermansia, you can help promote the growth of this beneficial bacteria in your gut, leading to better digestion and overall wellness.
In addition to Akkermansia, this supplement also contains prebiotic inulin fiber, which helps to feed and nourish the good bacteria in your gut. This combination of probiotics and prebiotics can help to improve gut function, reduce inflammation, and support weight management.
Furthermore, Akkermansia has been found to increase the production of GLP-1, a hormone that helps regulate blood sugar levels and promote satiety. By supporting the production of GLP-1, this supplement can help to improve insulin sensitivity and control cravings, making it easier to maintain a healthy weight.
If you’re looking to support your gut health and improve your overall well-being, consider adding Akkermansia Probiotic Supplement with Prebiotic Inulin Fiber to your daily routine. Your gut will thank you!
#Akkermansia #Probiotic #Supplement #Prebiotic #Inulin #Fiber #GLP1 #Production

