Your cart is currently empty!
Tag: Risks
Ozempic and similar weight loss drugs may lower risk of 42 health conditions, but also pose risks
Several years ago, a little-known drug named Ozempic – previously used only to treat diabetes – emerged as a promising new drug for weight management.
The Food and Drug Administration’s approval of Ozempic in 2021 for weight loss treatment ushered in a new era for the class of drugs called glucagon-like peptide-1 agonists, or GLP-1.
Today, GLP-1 drugs, including Wegovy, Mounjaro and Zepbound, have become household names and key tools in the fight against obesity: 1 in 8 American adults say they have used a GLP-1 drug, and forecasts show that by 2030, 1 in 10 Americans will likely be using these medications.
Now, research from my lab and others suggests that GLP-1 drugs could help treat dozens of other ailments as well, including cognitive issues and addiction problems. However, my colleagues and I also found previously unidentified risks.
I am a physician-scientist and I direct a clinical epidemiology center focused on addressing public health’s most urgent questions. My team works to address critical knowledge gaps about COVID-19, long COVID, influenza, vaccines, effectiveness and risks of commonly used drugs, and more.
On Jan. 20, 2025, my team published a study of more than 2.4 million people that evaluated the risks and benefits of GLP-1 drugs across 175 possible health outcomes. We found that these drugs lowered risks of 42 health outcomes, nearly a quarter of the total that we analyzed. These include neurocognitive disorders such as Alzheimer’s disease and dementia, substance use and addiction disorders, clotting disorders and several other conditions.
Unfortunately, we also found that GLP-1 drugs come with significant side effects and increase the risk of 19 health conditions we studied, such as gastrointestinal issues, kidney stones and acute pancreatitis, in which the pancreas becomes inflamed and dysfunctional.
Initially, GLP-1 drugs were developed to treat diabetes. Cognitive benefits
One of the most important health benefits we found was that the GLP-1 drugs lowered the risk of neurodegenerative disorders, including Alzheimer’s disease and dementia. These findings align with other research, including evidence from preclinical studies showing that these drugs may reduce inflammation in the brain and enhance the brain’s ability to form and strengthen connections between its cells, improving how they communicate with one another. These effects contribute to mitigating cognitive decline.
Two other key studies have shown that patients treated with a GLP-1 drug for diabetes had a lower risk of dementia.
All of these studies strongly point to a potential therapeutic use of GLP-1 drugs in treatment of the cognitive decline. Ongoing randomized trials – the gold standard for evaluating new uses of drugs – are looking at the effects of GLP-1 drugs in early Alzheimer’s disease, with results expected later in 2025.
Curbing addiction and suicidal ideation
GLP-1 drugs have also demonstrated potential in reducing risks of several substance use disorders such as those involving alcohol, tobacco, cannabis, opioids and stimulants. This may be due to the ability of these drugs to modulate reward pathways, impulse control and inflammatory processes in the brain.
The effectiveness of GLP-1 drugs in curbing addictive behavior may explain their spectacular success in treating obesity, a chronic disease state that many have suggested is indeed a food addiction disorder.
Our study demonstrated a reduced risk of suicidal thoughts and self-harm among people using GLP-1 drugs. This finding is particularly significant given earlier reports of suicidal thoughts and self-injury in people using GLP-1 drugs. In response to those reports, the European Medicines Agency conducted a review of all available data and concluded that there was no evidence of increased risk of suicidality in people using GLP-1 drugs.
Now at least two studies, including our own, show that GLP-1 drugs actually reduce the risk of suicidality.
Other benefits
In addition to the well-documented effects of GLP-1 drugs in reducing risks of adverse cardiovascular and kidney outcomes, our study shows a significant effect in reducing risk of blood clotting as well as deep vein thrombosis and pulmonary embolism.
One puzzling finding in our study is the reduced risk of infectious diseases such as pneumonia and sepsis. Our data complements another recent study that came to a similar conclusion showing that GLP-1 drugs reduced risk of cardiovascular death and death due to infectious causes, primarily COVID-19.
This is especially important since COVID-19 is regarded as a significant cardiovascular risk factor. Whether GLP-1 drugs completely offset the increased risk of cardiovascular disease associated with COVID-19 needs to be thoroughly evaluated.
GLP-1 drugs may also be useful in treating fatty liver disease and conditions ranging from asthma to chronic obstructive pulmonary disease, sleep apnea, osteoarthritis, depression and eye disorders.
Some doctors are prescribing GLP-1 drugs to help with fertility issues. Risks and challenges
Despite their broad therapeutic potential, GLP-1 drugs are not without risks.
Gastrointestinal issues, such as nausea, vomiting, constipation and gastroesophageal reflux disease are among the most common adverse effects associated with GLP-1 drugs.
Our study also identified other risks, including low blood pressure, sleep problems, headaches, formation of kidney stones, and gall bladder disease and diseases associated with the bile ducts. We also saw increased risks of drug-induced inflammation of the kidneys and pancreas – both serious conditions that can result in long-term health problems. These findings underscore the importance of careful monitoring in people who are taking GLP-1 medications.
A significant challenge with using GLP-1 drugs is the high rates at which patients stop using them, often driven by their exorbitant cost or the emergence of adverse effects. Discontinuation can lead to rapid weight gain.
That’s a problem, because obesity is a chronic disease. GLP-1 drugs provide effective treatment but do not address the underlying causes of obesity and metabolic dysfunction. As a result, GLP-1 drugs need to be taken long term to sustain their effectiveness and prevent rebound weight gain.
In addition, many questions remain about the long-term effectiveness and risks of these drugs as well as whether there are differences between GLP-1 formulations. Addressing these questions is critical to guide clinical practice.
Ozempic and similar weight loss drugs have been found to potentially lower the risk of 42 different health conditions, according to recent studies. These medications have shown promising results in helping individuals shed excess pounds and improve their overall health. However, it is important to note that these drugs also come with risks that should be carefully considered before beginning treatment.While the benefits of weight loss medications such as Ozempic are clear, there are also potential side effects and risks associated with their use. These can include nausea, diarrhea, constipation, headaches, and potential interactions with other medications. In some cases, these drugs have also been linked to more serious side effects such as pancreatitis, kidney damage, and thyroid cancer.
Before starting any weight loss medication, it is crucial to consult with a healthcare provider to discuss the potential risks and benefits. It is important to weigh these factors carefully and make an informed decision based on individual health needs and medical history.
Overall, while Ozempic and similar weight loss drugs may offer significant health benefits, it is essential to approach their use with caution and awareness of the potential risks involved. Consulting with a healthcare professional is key to ensuring safe and effective treatment.
Tags:
Ozempic, weight loss drugs, health conditions, lower risk, risks, medication, side effects, benefits, safety, medical research, obesity, diabetes, cardiovascular health, potential risks, drug interactions, weight management, chronic conditions, health risks, drug safety, clinical trials, weight loss treatment, health benefits
#Ozempic #similar #weight #loss #drugs #risk #health #conditions #pose #risksOzempic’s health benefits keep growing, but are the risks worth it?
One in eight adults in the U.S. has taken Ozempic or another type of GLP-1 drug, surveys show — and now a major new study has revealed a long list of benefits and some little-known risks.
Glucagon-like peptide-1 receptor (GLP-1) agonists — which contain either semaglutide or liraglutide — are prescribed to treat type 2 diabetes and obesity, but previous studies have linked the drugs to other, unexpected benefits.
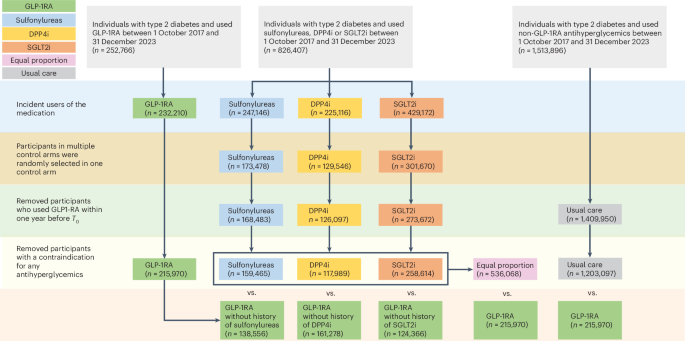
Researchers at Washington University School of Medicine in St. Louis and the Veterans Affairs (VA) St. Louis Health Care System studied the health outcomes of more than two million veterans with diabetes who took Ozempic or another type of GLP-1 drug between Oct. 1, 2017 and Dec. 31, 2023, according to a university press release.
OZEMPIC ‘MICRODOSING’ IS THE NEW WEIGHT-LOSS TREND
They then compared those outcomes to veterans who took non-GLP-1 diabetes medications.

One in eight adults in the U.S. has taken Ozempic or another type of GLP-1 drug, surveys show. (iStock)
The study, published in the journal Nature on Jan. 20, identified “widespread associations” between Ozempic and some significant health benefits.
Those benefits included reduced risks of neurocognitive disorders like Alzheimer’s and dementia, seizures, and addiction to harmful substances such as alcohol, cannabis, stimulants and opioids.
Other positive health effects included lower risks of suicidal ideation, self-harm, bulimia and psychotic disorders such as schizophrenia.
OZEMPIC COULD HELP REDUCE ALZHEIMER’S RISK FOR SOME, STUDY SUGGESTS: ‘SHIFTING THE PARADIGM’
“GLP-1s have many benefits for at least 42 conditions beyond weight loss – from addiction disorders to memory/cognition problems, blood clots, and infections,” lead study author Ziyad Al-Aly, clinical epidemiologist at Washington University in St. Louis, told Fox News Digital.
Ozempic and other GLP-1 drugs act on receptors in the brain that are involved in impulse control, reward and addiction, Al-Aly noted, which could explain why they are effective in reducing cravings for food and addictive substances.
“GLP-1s have many benefits for at least 42 conditions beyond weight loss.”
“These drugs also reduce inflammation in the brain and result in weight loss; both of these factors may improve brain health and explain the reduced risk of conditions like Alzheimer’s disease and dementia,” he added.
They also found, however, that GLP-1 drugs were linked to several adverse side effects.

Novo Nordisk is the maker of Ozempic, which is approved for type 2 diabetes treatment and reduced risk of cardiovascular events. (Getty Images)
The risk of gastrointestinal problems — including nausea, vomiting, diarrhea and a rarer paralysis of the stomach — were widely known before this new study, the researcher noted.
The new finding, however, was that these drugs can negatively affect the pancreas and kidneys. They were also linked to a higher chance of developing arthritis.
DIABETES AND WEIGHT LOSS DRUGS SHOWN TO REDUCE ALCOHOL-RELATED HOSPITALIZATIONS, STUDY FINDS
“While these adverse effects are uncommon, they can be very serious; physicians must be vigilant for signs of pancreatitis (inflammation of the pancreas) and monitor kidney function among people taking GLP-1RA medications,” Al-Aly stated in the press release.
“Kidney problems can occur without symptoms until the condition is at an advanced stage with limited treatment options.”

Decreased obesity has long been linked with less inflammation in every body system, an expert pointed out. (iStock)
The study, which was funded by the U.S. Department of Veterans Affairs, did have some limitations, the researchers acknowledged.
“This is a discovery approach involving more than two million people and is not a randomized trial,” Al-Aly told Fox News Digital.
“People with real weight loss are happier overall — they feel empowered.”
Seth Kipnis, MD, medical director of bariatric and robotic surgery at Hackensack Meridian Jersey Shore University Medical Center, was not involved in the study but said it confirms what he has seen in his own clinical practice.
“People with real weight loss are happier overall — they feel empowered that they can finally control their weight,” he said in a statement sent to Fox News Digital.

The risk of gastrointestinal problems — including nausea, vomiting, diarrhea and a rarer paralysis of the stomach — were widely known before this new study. (iStock)
Decreased obesity has long been linked with less inflammation in every body system, Kipnis pointed out.
“GI problems and renal problems will always be seen if people are not eating correctly and maintaining hydration,” he said. “If you take these medications without nutritional education, they can be harmful.”
Recommendations and predictions
Based on the findings, the researchers noted, people should be aware that these drugs have not only a “broad beneficial profile,” but also important risks.
“People should use the information to discuss with their provider whether GLP-1 is the right medication for them,” Al-Aly recommended.
“A person with a lot of GI issues may find these meds intolerable.”
“A person who is trying to lose weight and quit smoking or drinking may find GLP-1s especially useful – helping hit two birds with one stone — but a person with a lot of GI (gastrointestinal) issues may find these meds intolerable,” he went on.
“Each person’s health profile is different. Pros and cons should be discussed with the provider.”
CHEAP OZEMPIC KNOCK-OFFS HAVE RISEN IN POPULARITY
Given their effectiveness at causing weight loss and improving health, GLP-1 drugs will likely become more common and increase in use, Hackensack’s Kipnis predicted.
“We have been prescribing many long-term drugs for hypertension, heart disease, high cholesterol, diabetes, arthritis, reflux and many other diseases without hesitation,” he said.

The new study revealed that GLP-1 drugs could negatively affect the pancreas and kidneys. (iStock)
“This new class of drug, as it lowers obesity, has the potential to lower the use of every other drug that is used to treat obesity-related conditions.”
Not every doctor should prescribe these medications, however, according to Kipnis.
“Doctors with weight management programs and nutrition education programs would likely have better outcomes and fewer side effects,” the doctor said.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Novo Nordisk, the maker of Ozempic (approved for type 2 diabetes treatment and reduced risk of cardiovascular events) and Wegovy (approved for weight management and reduced risk of cardiovascular events), provided the following statement to Fox News Digital.
CLICK HERE TO GET THE FOX NEWS APP
“Semaglutide has helped millions of people fight chronic diseases such as type 2 diabetes, cardiovascular disease and obesity. As we look to help even more people with chronic diseases, we are exploring the potential of semaglutide in several other disease states.”

Wegovy, also made by Novo Nordisk, is FDA-approved for weight management and reduced risk of cardiovascular events. (Steve Christo – Corbis/Corbis via Getty Images)
“Patient safety is of utmost importance to Novo Nordisk,” the company also said.
For more Health articles, visit www.foxnews.com/health
“The known risks and benefits of semaglutide medicines are described in their FDA-approved product labeling, and we work closely with authorities and regulatory bodies worldwide to continuously monitor the safety profile of our products.”

“Patient safety is of utmost importance,” said Novo Nordisk in a statement to Fox News Digital. (iStock)
The company added that the “totality of data” from previous studies “provides reassurance of the safety profile of semaglutide.”
Ozempic, a popular medication used to treat type 2 diabetes, has been making waves in the medical community for its impressive health benefits. From lowering blood sugar levels to aiding in weight loss, Ozempic has been proven to be an effective tool in managing diabetes.However, as with any medication, there are potential risks and side effects to consider. Some common side effects of Ozempic include nausea, diarrhea, and stomach pain. More serious risks include pancreatitis and thyroid cancer, although these are rare.
So, the question remains: are the potential risks of Ozempic worth the health benefits it provides? It ultimately depends on the individual and their specific health needs. It is important to weigh the benefits and risks with your healthcare provider to determine if Ozempic is the right choice for you.
What are your thoughts on Ozempic? Have you or someone you know experienced positive or negative effects from taking this medication? Share your experiences in the comments below.
Tags:
- Ozempic
- Health benefits
- Risks
- Diabetes medication
- Side effects
- Ozempic benefits
- Weight loss
- Blood sugar control
- Type 2 diabetes
- Medication risks
#Ozempics #health #benefits #growing #risks #worth
Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists

Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists

Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists
Mapping the effectiveness and risks of GLP-1 receptor agonists
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Watanabe, J. H., Kwon, J., Nan, B. & Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 64, 133–138 (2024).
Hegland, T.A., Fang, Z. & Bucher, K. GLP-1 medication use for type 2 diabetes has soared.JAMA 332, 952–953 (2024).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Vidal, J., Flores, L., Jiménez, A., Pané, A. & de Hollanda, A. What is the evidence regarding the safety of new obesity pharmacotherapies. Int. J. Obes. https://doi.org/10.1038/s41366-024-01488-5 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Laurindo, L. F. et al. GLP-1a: going beyond traditional use. Int. J. Mol. Sci. 23, 739 (2022).
Rubin, R. Could GLP-1 receptor agonists like semaglutide treat addiction, Alzheimer disease, and other conditions? JAMA 331, 1519–1521 (2024).
Wang, W. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 15, 4548 (2024).
Wang, W. et al. Association of semaglutide with tobacco use disorder in patients with type 2 diabetes: target trial emulation using real-world data. Ann. Intern. Med. 177, 1016–1027 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Lenharo, M. Why do obesity drugs seem to treat so many other ailments? Nature 633, 758–760 (2024).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131, 372–379 (2022).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Yammine, L., Balderas, J. C., Weaver, M. F. & Schmitz, J. M. Feasibility of exenatide, a GLP-1R agonist, for treating cocaine use disorder: a case series study. J. Addict. Med. 17, 481–484 (2023).
Angarita, G. A. et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 221, 108614 (2021).
Dixit, T. S., Sharma, A. N., Lucot, J. B. & Elased, K. M. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol. Behav. 114−115, 38–41 (2013).
Gunturu, S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J. Psychol. Med. 46, 193–195 (2024).
López-Ojeda, W. & Hurley, R. A. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4–A86 (2024).
Flintoff, J., Kesby, J. P., Siskind, D. & Burne, T. H. J. Treating cognitive impairment in schizophrenia with GLP-1RAs: an overview of their therapeutic potential. Expert Opin. Investig. Drugs 30, 877–891 (2021).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8−11 April 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024 (12 April 2024).
Du, H., Meng, X., Yao, Y. & Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 13, 1033479 (2022).
Mehan, S. et al. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 12, 31–59 (2022).
Colin, I. M., Szczepanski, L. W., Gérard, A. C. & Elosegi, J. A. Emerging evidence for the use of antidiabetic drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s disease. touchREV. Endocrinol. 19, 16–24 (2023).
Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature 626, 246 (2024).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 8, e12268 (2022).
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Atri, A. et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating the neuroprotective effects of semaglutide in early Alzheimer’s disease. Alzheimer’s Dement. 18, e062415 (2022).
Manavi, M. A. Neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogues in epilepsy and associated comorbidities. Neuropeptides 94, 102250 (2022).
Wang, L. et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice. Int. J. Mol. Med. 48, 219 (2021).
Hussein, A. M. et al. Effects of GLP-1 receptor activation on a pentylenetetrazole−kindling rat model. Brain Sci. 9, 108 (2019).
Liu, S. et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of Dravet syndrome. Front. Pharmacol. 11, 136 (2020).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Jia, G., Aroor, A. R. & Sowers, J. R. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes 65, 1487–1489 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Sternkopf, M. et al. Native, intact glucagon-like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arter. Thromb. Vasc. Biol. 40, e65–e77 (2020).
Steven, S. et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 174, 1620–1632 (2017).
Cameron-Vendrig, A. et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65, 1714–1723 (2016).
Zhang, Y., Chen, R., Jia, Y., Chen, M. & Shuai, Z. Effects of exenatide on coagulation and platelet aggregation in patients with type 2 diabetes. Drug Des. Devel. Ther. 15, 3027–3040 (2021).
Horvei, L. D., Brækkan, S. K. & Hansen, J. B. Weight change and risk of venous thromboembolism: the Tromsø study. PLoS ONE 11, e0168878 (2016).
de Lemos, J. A. et al. Tirzepatide reduces 24-hour ambulatory blood pressure in adults with body mass index ≥27 kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 81, e41–e43 (2024).
Goodwill, A. G. et al. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev. Endocr. Metab. Disord. 15, 209–217 (2014).
Ribeiro-Silva, J. C., Tavares, C. A. M. & Girardi, A. C. C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: a mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 69, 102355 (2023).
Goud, A., Zhong, J., Peters, M., Brook, R. D. & Rajagopalan, S. GLP-1 agonists and blood pressure: a review of the evidence. Curr. Hypertens. Rep. 18, 16 (2016).
Yang, F. et al. GLP-1 receptor: a new target for sepsis. Front. Pharmacol. 12, 706908 (2021).
Helmstädter, J. et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 10, 1175 (2021).
Yi, H. et al. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp. Neurol. 363, 114348 (2023).
Scirica, B. et al. The effect of semaglutide on mortality and COVID-19–related deaths.JACC 84, 1632–1642 (2024).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Yu, M. et al. The relationship between the use of GLP-1 receptor agonists and the incidence of respiratory illness: a meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 15, 164 (2023).
Altintas Dogan, A. D. et al. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 405–414 (2022).
Foer, D. et al. Association of GLP-1 receptor agonists with chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes. Am. J. Respir. Crit. Care Med. 208, 1088–1100 (2023).
Pradhan, R. et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ 379, e071380 (2022).
Yeo, Y.H. et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use.Gastroenterology 167, 402–404 (2024).
Dixit, A. A., Bateman, B. T., Hawn, M. T., Odden, M. C. & Sun, E. C. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA 331, 1672–1673 (2024).
Wang, W. et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 129–137 (2023).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity—from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-Delta, Delta, and Omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Cai, M., Xie, Y., Topol, E. J. & Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 30, 1564–1573 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14, 983 (2023).
Xu, E., Xie, Y. & Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 11, 120–128 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect. Dis. 24, 239–255 (2024).
Al-Aly, Z. & Topol, E. Solving the puzzle of long Covid. Science 383, 830–832 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Xie, Y. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J. Am. Soc. Nephrol. 27, 3153–3163 (2016).
Xie, Y. et al. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7, e015735 (2017).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 91, 1482–1494 (2017).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 93, 741–752 (2018).
Maynard, C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3783-notes.pdf (2017).
Cai, M. et al. Temporal trends in incidence rates of lower extremity amputation and associated risk factors among patients using Veterans Health Administration services from 2008 to 2018. JAMA Netw. Open 4, e2033953 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 11, 644–656 (2023).
Xie, Y. et al. Clinical implications of estimated glomerular filtration rate dip following sodium−glucose cotransporter-2 inhibitor initiation on cardiovascular and kidney outcomes. J. Am. Heart Assoc. 10, e020237 (2021).
Xie, Y. et al. Comparative effectiveness of sodium−glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 181, 1043–1053 (2021).
Xie, Y. et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43, 2859–2869 (2020).
Xie, Y. et al. Comparative effectiveness of the sodium−glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43, 2785–2795 (2020).
Xie, Y., Choi, T. & Al-Aly, Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19.JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 381, e073312 (2023).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ 381, e074572 (2023).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Schneeweiss, S. Automated data-adaptive analytics for electronic healthcare data to study causal treatment effects. Clin. Epidemiol. 10, 771–788 (2018).
Schneeweiss, S. et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20, 512–522 (2009).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009).
Crump, R. K., Hotz, V. J., Imbens, G. W. & Mitnik, O. A. Dealing with limited overlap in estimation of average treatment effects. Biometrika 96, 187–199 (2009).
Hernan, M. A. & Robins, J. M. Causal Inference: What If (CRC Press, 2010).
Uno, H. et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32, 2380–2385 (2014).
Andersen, P. K., Hansen, M. G. & Klein, J. P. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 10, 335–350 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
GLP-1 receptor agonists are a class of medications commonly used to treat type 2 diabetes by stimulating insulin production and reducing blood sugar levels. However, like any medication, there are potential risks and side effects associated with their use.In this post, we will explore the effectiveness of GLP-1 receptor agonists in managing type 2 diabetes, as well as the potential risks and side effects that patients should be aware of.
Effectiveness of GLP-1 receptor agonists:
Studies have shown that GLP-1 receptor agonists are highly effective in lowering blood sugar levels in patients with type 2 diabetes. They work by stimulating the release of insulin from the pancreas, slowing down digestion, and reducing the production of glucose in the liver.
In addition to lowering blood sugar levels, GLP-1 receptor agonists have also been shown to promote weight loss in patients with type 2 diabetes. This is because they can reduce appetite and increase feelings of fullness, leading to a decrease in caloric intake.
Risks and side effects of GLP-1 receptor agonists:
While GLP-1 receptor agonists are generally well-tolerated, there are some potential risks and side effects that patients should be aware of. These can include:
– Nausea and vomiting
– Diarrhea
– Hypoglycemia (low blood sugar)
– Pancreatitis
– Thyroid tumors
– Allergic reactionsIt’s important for patients to discuss the potential risks and benefits of GLP-1 receptor agonists with their healthcare provider before starting treatment. Additionally, patients should be monitored regularly for any signs of side effects or complications.
Overall, GLP-1 receptor agonists are a valuable treatment option for patients with type 2 diabetes, but it’s important to weigh the potential risks and benefits before starting treatment. By mapping out the effectiveness and risks of these medications, patients can make informed decisions about their diabetes management.
Tags:
- GLP-1 receptor agonists
- Effectiveness of GLP-1 receptor agonists
- Risks of GLP-1 receptor agonists
- GLP-1 agonist benefits
- GLP-1 receptor agonist safety
- GLP-1 agonist side effects
- GLP-1 agonist risk assessment
- GLP-1 receptor agonist efficacy
- GLP-1 agonist comparison
- GLP-1 agonist research findings
#Mapping #effectiveness #risks #GLP1 #receptor #agonists
Single-digit temperature can cause serious health risks
The Susquehanna Valley is freezing and when temperatures dip to the single digits, health risks are heightened. Doctors with WellSpan Health say without proper gear, frostbite can happen in just a matter of minutes. Frostbite is serious and can lead to gangrene, which is the death of body tissue. If this happens, a part of your body may have to be amputated.Parents may be walking their children to school in the early morning hours.Dr. Hersh Shah, who is an urgent care physician with WellSpan Health, said it’s important for parents to ensure their kids wear a long sleeve, a jacket and multiple layers as they are coming to and from school.”They might feel uncomfortable saying ‘Oh this feels really warm. I’m getting hot or sweaty,’” Shah said. “That may be the case inside but, when you’re looking at a drastic temperature change going indoors and outdoors, I say bundle them up.” Cold weather can also have negative effects on those battling an illness or who have preexisting conditions.Low temperatures can put stress on the heart for those with preexisting heart conditions, which could increase the risk of heart attacks. The weather can also make it harder to breathe, making symptoms for those with respiratory illnesses like the flu more severe.Some who are most at risk of respiratory failure are those with existing respiratory issues like asthma. Dr. Shah said it’s important to keep an inhaler on hand at all times.”If you have a patient who has been a long-term smoker if they have a history of recurring bronchitis… that temperature itself can cause a lot of irritation to the lungs,” he said. “It can cause contraction within the lung tissue, and that can trigger attacks where you get shorter breath really quickly or feelings of chest tightness where you just are not able to get air in.”Doctors are also concerned with slippery icy surfaces in single-digit temperatures.Shah said during this time, it’s not uncommon to treat people for injuries after slipping and falling, which could be severe for children and older adults. For this reason, doctors recommend wearing boots or shoes with a good grip.
LEBANON, Pa. —The Susquehanna Valley is freezing and when temperatures dip to the single digits, health risks are heightened.
Doctors with WellSpan Health say without proper gear, frostbite can happen in just a matter of minutes.
Frostbite is serious and can lead to gangrene, which is the death of body tissue. If this happens, a part of your body may have to be amputated.
Parents may be walking their children to school in the early morning hours.
Dr. Hersh Shah, who is an urgent care physician with WellSpan Health, said it’s important for parents to ensure their kids wear a long sleeve, a jacket and multiple layers as they are coming to and from school.
“They might feel uncomfortable saying ‘Oh this feels really warm. I’m getting hot or sweaty,’” Shah said. “That may be the case inside but, when you’re looking at a drastic temperature change going indoors and outdoors, I say bundle them up.”
Cold weather can also have negative effects on those battling an illness or who have preexisting conditions.
Low temperatures can put stress on the heart for those with preexisting heart conditions, which could increase the risk of heart attacks.
The weather can also make it harder to breathe, making symptoms for those with respiratory illnesses like the flu more severe.
Some who are most at risk of respiratory failure are those with existing respiratory issues like asthma. Dr. Shah said it’s important to keep an inhaler on hand at all times.
“If you have a patient who has been a long-term smoker if they have a history of recurring bronchitis… that temperature itself can cause a lot of irritation to the lungs,” he said. “It can cause contraction within the lung tissue, and that can trigger attacks where you get shorter breath really quickly or feelings of chest tightness where you just are not able to get air in.”
Doctors are also concerned with slippery icy surfaces in single-digit temperatures.
Shah said during this time, it’s not uncommon to treat people for injuries after slipping and falling, which could be severe for children and older adults. For this reason, doctors recommend wearing boots or shoes with a good grip.
As the winter months approach and temperatures drop, it’s important to be aware of the potential health risks that come with single-digit temperatures. Exposure to cold temperatures can have serious consequences on your health, especially if you are not properly prepared.Hypothermia is a common risk when temperatures are in the single digits. This occurs when your body loses heat faster than it can produce it, causing your body temperature to drop to dangerous levels. Symptoms of hypothermia include shivering, confusion, slurred speech, and drowsiness. If left untreated, hypothermia can be life-threatening.
Frostbite is another concern when temperatures are extremely low. This occurs when skin and tissue freeze due to exposure to cold temperatures. Symptoms of frostbite include numbness, tingling, and discoloration of the skin. Severe cases of frostbite can result in permanent damage to the affected area.
To protect yourself from these health risks, it’s important to dress warmly and in layers when going outside in single-digit temperatures. Make sure to wear a hat, gloves, and insulated clothing to keep your body heat trapped. Limit your time outdoors and seek shelter if you start to feel cold.
If you or someone you know is experiencing symptoms of hypothermia or frostbite, seek medical attention immediately. Remember that single-digit temperatures can be dangerous, so take precautions to keep yourself safe and warm during the winter months.
Tags:
- Cold weather health risks
- Single-digit temperature dangers
- Winter health hazards
- Extreme cold health effects
- Cold weather safety tips
- Hypothermia prevention strategies
- Frostbite warning signs
- Stay safe in frigid temperatures
- Cold weather health precautions
- Protecting your health in subzero temperatures
#Singledigit #temperature #health #risks
I was prescribed a common antibiotic and wasn’t told of the risks… I’m now disabled and need 24/7 care
A woman rendered permanently disabled and wheelchair-bound after being prescribed a common antibiotic in 2021 is warning others about its side effects.
An avid runner and fitness fanatic at the time, Talia Smith, 45, was prescribed ciprofloxacin, or Cipro for short, to treat a urinary tract infection in April 2021.
What happened next felt like ‘a bomb going off’ in her body — after three pills, sharp pains like she was being electrocuted shot up from her feet to her legs.
Her muscles became so stiff that she couldn’t move. She went to the hospital but claims doctors sent her home and told her to take ibuprofen.
Eventually, she could no longer shower by herself or chew and swallow food unless it was pureed first. Because of her own complex health needs and those of her husband, a disabled veteran, they can no longer live in the same home.
When Smith’s story first broke, it inspired more Americans to come forward with their harrowing experiences on Cipro.
Yet, the drug continued to be prescribed to millions of Americans every year without doctors warning about its links to a devastating condition that causes irreparable nerve damage.
But now, almost four years on, the CDC will now formally recognize the side effect known colloquially as ‘floxing.’


Talia Smith, pictured with her husband (left), was active and healthy before being prescribed Cipro to treat a UTI – now she’s disabled and needs constant care

When Mrs Smith moved into hospice care she couldn’t swallow solid food, walk on her own, or lift her arms over her head
Fluoroquinolones, whether taken by mouth or inhaled, can cause serious side effects that may be disabling, long-lasting, and possibly permanent. These side effects are estimated to affect at least 1 to 10 people out of every 10,000 who use these medications.
Using her harrowing health journey as a catalyst for advocacy, Smith and doctors successfully pressed the CDC to get the condition officially recognized as a diagnosable, reportable condition, helping to bring more awareness to the issue and making it easier to track. That policy came into effect in July 2024.
The CDC’s formal recognition was ‘something like landing on the moon,’ according to Dr Stefan Pieper, who has treated around 1,500 patients with fluoroquinolone poisoning at his practice in Germany and presented the proposal to the CDC.
Smith says in her case, she asked her doctor about the drug’s possible side effects and its strength but was told the medication was safe and widely prescribed.
However, the doctor did not mention the FDA‘s series of Black Box warnings, the first being issued in 2008, about the risks of tendonitis, nerve damage causing numbness, pain and tingling, seizures, tremors, and several others.
Mrs Smith said: ‘I took the antibiotic. Three pills in, I couldn’t walk. I started having pains all over my body. My vision actually changed in this time. I had problems swallowing.’
Fluoroquinolones, the class of antibiotics to which Cipro belongs, comes with a laundry list of potentially devastating side effects from tendon rupture and muscle wasting to nerve damage and aortic tears. Despite the risks, it’s prescribed to roughly 15 million Americans every year.
When Mrs Smith went to her local emergency department in Norwood, Massachusetts, one of the first questions a doctor asked her was, ‘Are you taking Cipro?’
It was then that she learned of the Black Box warnings, the most stringent the FDA could hand down, and that many of her symptoms were there – tremors, sharp, shooting pains, dizziness, and muscle weakness.
Though she stopped taking the medicine after two days, the damage was done.
She got worse by the week.


Prior to taking Cipro, Mrs Smith (pictured left, with her sister) exercised regularly and seldom needed medication. She rarely fell ill and only visited the doctor for her annual checkups
Five months later, she was in hospice care, weighing 60 lbs.
‘The nerve pain was ridiculous, just constant nerve pain,’ she said.
She added: ‘And as for my life, it’s flipped upside down. I can’t even take care of myself. I’m on palliative care. I need care 24/7.’
Cipro is a fluoroquinolone antibiotic, and while it targets harmful bacteria, it can also target human cells, including nerve cells.
It can interfere with the function of mitochondria in cells, which govern energy production and cellular repair. Stress on cells harms nerves, disrupting their normal signaling pathways.
It can also affect how nerves send signals by interfering with tiny pathways, called ion channels, that help nerves communicate. This disruption can cause unusual sensations, like pain or tingling.

Mrs Smith working out before her reaction

Previously a caregiver to her veteran husband, Mrs Smith was forced to stop when her own debilitating illness emerged, resulting in the couple living apart
The FDA has received reports of hundreds of thousands of serious adverse events associated with fluoroquinolones from more than 60,000 patients since the 1980s.
One recent case involves 61-year-old actor Rick Zingale from New Jersey, who played Don Miguel in Rambo: Last Blood (2019). In 2022, he was hospitalized with symptoms doctors initially believed to be bronchitis and pneumonia.
Zingale was given an IV drip of the fluoroquinolone antibiotic levofloxacin to treat what was thought to be a bacterial lung infection. However, he later learned that he was actually suffering from heart failure, making the antibiotics ineffective.
Unfortunately, the drugs caused him life-altering complications. He developed a red, swollen mass near his right collarbone and experiences pain that ‘shoots down’ his neck and right arm. Additionally, he has developed arthritis in his right hand, which he believes is linked to the medication.
He said: ‘I’m defeated… because I have these horrible symptoms and I don’t know what’s going on… I’m absolutely distraught.’
Another person affected is 44-year-old Mindy Tautfest from Oklahoma City.
In 2016, the former ICU nurse had surgery to remove her appendix, she shared with DailyMail.com.
About three weeks later, doctors informed her that the internal stitches had become infected and prescribed a week-long course of ciprofloxacin, also known as Cipro.
With her extensive experience in prescribing this drug for similar infections, Mrs. Tautfest wasn’t initially concerned. However, after starting the medication, she began feeling unusually off. “I can’t describe the weird feeling, I just didn’t feel quite right,” she explained.
Just two months after beginning the drug, she suffered a vertebral artery dissection, which is a tear in the artery that supplies blood to the brain. Unfortunately, in her case, it led to a stroke.

Mindy Tautfest with her family shortly before the vertebral dissection occurred, which she believes is due to the Cipro she was prescribed
‘It was like a gunshot erupted inside my head,’ she said.
‘I felt the back of my head and couldn’t feel any blood. That’s when I realized that it was probably some type of a brain aneurysm inside my head that had ruptured.
‘It was like a peeling away – I call it an electrical avalanche – that rolled down my body. It just felt horrendous.’
Another one of many affected is 85-year-old John Sunderland Manousso from Texas, who visited his doctor in July 2023 for a recurring urinary tract infection (UTI).
His doctor prescribed a six-day course of levofloxacin. Just two days into taking the antibiotic, John began having difficulty walking.
His wife, Barbara, explained to DailyMail.com: ‘He said to me: “I’ve got knives coming up my legs.”’
‘He was wobbling like he’s on a boat, side to side, and obviously in pain.’
The couple began researching online and found a warning that the drug should not be given to people over 60 or those with motor issues. John had been diagnosed with vascular parkinsonism, which affects walking and balance, a condition they had discussed with the doctor before the prescription.

John Sunderland was previously an avid runner. His wife told DailyMail.com his legs tripled in size due to being so swollen after he took the antibiotic

John now cannot get around without a walker, and has been left with ‘terrible pain and balance issues’ which have caused falls, including one in which he tore his rotator cuff
They contacted the doctor, who assured them that the side effects would go away once John stopped taking the drug.
But things got worse. A few days later, John could ‘barely move,’ and his legs had swollen to more than three times their normal size.
At a gala in Newport, Rhode Island, a couple of weeks later, when John tried to stand up, he ‘screamed.’ It turned out that the tendon in his right leg had torn.
Tendon ruptures are a known side effect of fluoroquinolones.
Now, John can no longer walk without a walker and is dealing with “terrible pain and balance issues,” which have caused several falls, including one in which he tore his rotator cuff.
Doctors are prescribing Cipro less frequently than other antibiotics because of the life-altering consequences.
Overusing Cipro has also led to some bacteria becoming resistant to it, making infections caused by those bacteria harder to treat in the future. Better alternatives exist for common conditions, including UTIs and lung infections.
Mrs Smith said: ‘Make sure you actually need an antibiotic before taking one.
‘Antibiotics in the United States are so overprescribed and we’re very used to taking them if a doctor tells you a medication is safe. Double check, triple check, just to be sure.’
Being prescribed antibiotics is a common occurrence for many people when dealing with infections or illnesses. However, what happens when you are not informed of the potential risks associated with these medications?This was the case for me when I was prescribed a common antibiotic for a sinus infection. I was not told of the potential side effects or risks that could come with taking this medication. As a result, I suffered a severe adverse reaction that left me disabled and in need of 24/7 care.
The side effects of this antibiotic were debilitating, causing me to experience chronic pain, muscle weakness, and cognitive impairment. I now rely on others for basic tasks and require constant supervision and care.
It is crucial for healthcare providers to fully inform patients of the potential risks and side effects of any medication they are prescribed. In my case, not being made aware of these risks has had devastating consequences on my quality of life.
I urge everyone to advocate for their own health and ask questions about any medications they are prescribed. It is essential to be informed and aware of the potential risks so that you can make the best decisions for your health and well-being.
Tags:
- Antibiotic risks
- Common antibiotic side effects
- Disability from medication
- Prescription drug dangers
- Antibiotic complications
- 24/7 care needs
- Medical negligence consequences
- Antibiotic disability
- Patient rights awareness
- Medication safety awareness
#prescribed #common #antibiotic #wasnt #told #risks.. #disabled #care
What is an oligarchy? Biden warns the US risks centralizing power
WASHINGTON (AP) — President Joe Biden in his Wednesday farewell speech to the nation warned that American democracy was sliding into an “oligarchy” of tech billionaires. But what exactly is an oligarchy?
What is an oligarchy?
In short, an oligarchy is an elite few who control the government’s actions.
By using the pointedly negative term “oligarchy,” Biden equated this moment — when the world’s wealthiest men are feting President-elect Donald Trump — with some of history’s more brutal regimes.
Meta CEO Mark Zuckerberg is scheduled to cohost a Trump inaugural reception with wealthy Republican donors next week. Amazon Prime Video, which was founded by billionaire Jeff Bezos, got exclusive licensing rights to stream and theatrically release first lady Melania Trump ‘s new documentary.
Meta, Amazon and OpenAI CEO Sam Altman last month donated $1 million to Trump’s inauguration fund. And billionaire Elon Musk’s super PAC spent around $200 million to help elect Trump.
But Biden made a complicated assertion as both Republicans and Democrats have relied on Silicon Valley fortunes to boost their political ambitions.
What are the term’s origins?
Like many words in politics, oligarchy originates from Ancient Greek and quite literally means that few command. But unlike an aristocracy, an oligarchy is more closely tied to wealth than nobility and family lineage.
The philosopher Aristotle wrote in his book “Politics” that “democracy is safer and more free from civil strife than oligarchy; for in oligarchies two kinds of strife spring up, faction between different members of the oligarchy and also faction between the oligarchs and the people.”
What are some examples of oligarchies?
Multiple countries have been labeled oligarchies by academics. After the break-up of the Soviet Union in 1991, former state assets and other institutions came under the control of increasingly wealthy businessmen who became known as billionaire oligarchs.
The mix of profits and politics that began under then-Russian President Boris Yeltsin gave way to crackdowns by President Vladimir Putin, who has his own favored oligarchs and pledged to let them keep their fortunes so long as they are loyal to him.
With its legacy of colonialism and powerful families, the Philippines has been accused of being an oligarchy, with its former President Rodrigo Duterte claiming to have dismantled the system. Critics said he simply gave preferences to a different set of oligarchs.
Apartheid-era South Africa was also seen by some academics as having a white racial oligarchy.
Even before Biden’s speech, the rising wealth gap in the United States — as well as in China — raised concerns about whether the world’s two largest economies were becoming oligarchies.
Associated Press writers Lindsey Bahr and Dan Merica contributed reporting.
In recent remarks, President Joe Biden warned of the dangers of oligarchy and the risk of centralizing power in the United States. But what exactly is an oligarchy?An oligarchy is a form of government in which power is concentrated in the hands of a small, elite group of individuals or families. These individuals typically control the government, economy, and society, often to the detriment of the majority of the population.
Oligarchies can take various forms, including authoritarian regimes, plutocracies (where the wealthy hold power), and aristocracies (where power is inherited). In these systems, the ruling elite often use their wealth and influence to maintain their power and suppress dissent.
President Biden’s warning about the dangers of oligarchy comes at a time when income inequality in the US is at its highest levels in decades, and the influence of wealthy individuals and corporations in politics is a growing concern. Biden has called for policies to address these issues, such as increasing taxes on the wealthy and implementing campaign finance reform.
As the US grapples with the challenges of inequality and the concentration of power, it is important for citizens to remain vigilant and advocate for a more equitable and democratic society. Only by recognizing and addressing the dangers of oligarchy can we ensure a more just and fair system for all.
Tags:
oligarchy, US politics, centralization of power, Biden, government, democracy, political systems, power dynamics
#oligarchy #Biden #warns #risks #centralizing #power

